ACRIN-Contralateral-Breast-MR | ACRIN 6667
DOI: 10.7937/Q1EE-J082 | Data Citation Required | 2k Views | 2 Citations | Image Collection
| Location | Species | Subjects | Data Types | Cancer Types | Size | Status | Updated | |
|---|---|---|---|---|---|---|---|---|
| Breast | Human | 984 | MR, CR, Demographic, Diagnosis, Other | Breast Cancer | Clinical | Public, Complete | 2021/03/05 |
Summary
This dataset relates to NCI Clinical trial, "Magnetic Resonance Imaging in Women Recently Diagnosed With Unilateral Breast Cancer (ACRIN-6667)". The dataset consists of 984 patients but only 969 were included in the primary data analysis due to study criteria. Even after careful clinical and mammographic evaluation, cancer is found in the contralateral breast in up to 10% of women who have received treatment for unilateral breast cancer. ACRIN 6667 was conducted to determine whether magnetic resonance imaging (MRI) could improve on clinical breast examination and mammography in detecting contralateral breast cancer soon after the initial diagnosis of unilateral breast cancer. Additional information about the trial is available in the Study Protocol and Case Report Forms. METHODS A total of 969 women with a recent diagnosis of unilateral breast cancer and no abnormalities on mammographic and clinical examination of the contralateral breast underwent breast MRI. The diagnosis of MRI-detected cancer was confirmed by means of biopsy within 12 months after study entry. The absence of breast cancer was determined by means of biopsy, the absence of positive findings on repeat imaging and clinical examination, or both at 1 year of follow-up. RESULTS MRI detected clinically and mammographically occult breast cancer in the contralateral breast in 30 of 969 women who were enrolled in the study (3.1%). The sensitivity of MRI in the contralateral breast was 91%, and the specificity was 88%. The negative predictive value of MRI was 99%. A biopsy was performed on the basis of a positive MRI finding in 121 of the 969 women (12.5%), 30 of whom had specimens that were positive for cancer (24.8%); 18 of the 30 specimens were positive for invasive cancer. The mean diameter of the invasive tumors detected was 10.9 mm. The additional number of cancers detected was not influenced by breast density, menopausal status, or the histologic features of the primary tumor. CONCLUSIONS MRI can detect cancer in the contralateral breast that is missed by mammography and clinical examination at the time of the initial breast-cancer diagnosis.
Data Access
Version 1: Updated 2021/03/05
| Title | Data Type | Format | Access Points | Subjects | License | Metadata | |||
|---|---|---|---|---|---|---|---|---|---|
| Images | MR, CR | DICOM | Download requires NBIA Data Retriever |
984 | 1,103 | 10,184 | 626,782 | CC BY 4.0 | View |
| Clinical Data | Demographic, Diagnosis, Other | CSV, ZIP, and XLSX | CC BY 4.0 | — |
Additional Resources for this Dataset
The NCI Cancer Research Data Commons (CRDC) provides access to additional data and a cloud-based data science infrastructure that connects data sets with analytics tools to allow users to share, integrate, analyze, and visualize cancer research data.
- Imaging Data Commons (IDC) (Imaging Data)
The following external resources are not hosted or supported by TCIA, but may be useful to researchers utilizing this collection.
- ClinicalTrials.gov entry about the Trial NCT00058058, “Magnetic Resonance Imaging in Women Recently Diagnosed With Unilateral Breast Cancer (ACRIN-6667)”
Citations & Data Usage Policy
Data Citation Required: Users must abide by the TCIA Data Usage Policy and Restrictions. Attribution must include the following citation, including the Digital Object Identifier:
Data Citation |
|
|
Kinahan, P., Muzi, M., Bialecki, B., Herman, B., & Coombs, L. (2021). ACRIN-Contralateral-Breast-MR (ACRIN 6667) [Data set]. The Cancer Imaging Archive. https://doi.org/10.7937/Q1EE-J082 |
Detailed Description
Notes:
- There are data for 984 patients but only 969 were included in the primary data analysis due to study criteria.
- Due to a missing value in the type 1 Sequence Variant DICOM tag (0018,0021), it was decided to populate the missing tag values with “NONE” (the Sequence Variant is actually unknown but “Unknown” is not a valid value for this tag).
- Case Number 120 image data has been removed due to data corruption; clinical information is still present in clinical data spreadsheets.
Date Offsets:
All dates, like the visit date, are protected by presenting just the year; however, dates are also listed as offset days from the base date. The offset dates are used as a means of protecting patient information provided by the local sites in the original data, while allowing users to determine intervals between events. The standard DICOM date tags (i.e. birth dates, imaging study dates, etc.) have been de-identified so that all patients have a baseline study date of January 1, 1960. This falsified date represents the day patients were entered into trial database. The number of days between a subject’s longitudinal imaging studies are accurately preserved. A patient with a study performed on January 4, 1960 means the images were collected 3 days after the base date. For convenience, this calculation has been performed for all scans with the results inserted in DICOM tag (0012,0052) Longitudinal Temporal Offset From Event. This means an imaging study that took place on January 4, 1960 would contain a value of “3” in tag (0012,0052).
Overview of Clinical Data
The basic data flow for legacy ACRIN multi-center clinical trials was that all clinical information provided by the local imaging sites were contained in a series of forms. The form data submitted by local investigators to ACRIN during and after the trial, were manually encoded into the ACRIN CTMS (Clinical Trial Management System), and were cross-checked for accuracy by ECOG-ACRIN personnel. These ACRIN 6667 forms, filled out by the local sites, deliver information on imaging, clinical management of the patient and pathology/outcome variables, like dates of progression and survival, along with other critical information. The image data was initially anonymized while uploading from the local sites through TRIAD software and archived in a DICOM database at ACRIN.
After the trial accrual had ended, the clinical data was sent to the Brown statistical center, that is funded by NCI to provide support for ECOG-ACRIN clinical trials, specifically for analysis of the primary and sometimes secondary aims of the trial. The statisticians at Brown strip all the actual dates, names and other PHI from the CTMS data and create a .csv file for each form that has selected information useful for analysis of the trial data. A Form Dictionary file detailing all the forms used in the study accompanies the .csv data files. Additionally, the accompanying Form Dictionary file lists each element for each form that has been selected for data retention along with a description of each form element.
Extracting clinical (non-imaging) data example:
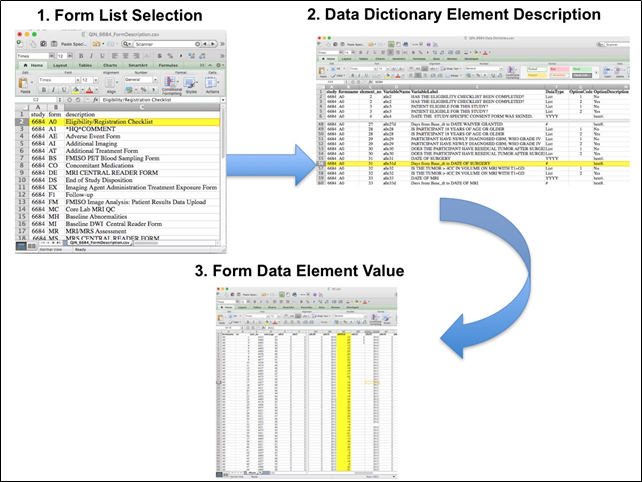
Beginning with the Form Dictionary.csv file, select the form with the desired information needed, such as form A0.csv the patient confirmation form. Next, using the form Dictionary.csv file (e.g. 6667_A0_Dictionary.csv), find the form elements listed for A0 (e.g., A0exx, where xx is the form element number). The file lists the form number, variable name, its description or label, the type of data, and, when applicable, the option codes and corresponding text values (option code:description pairs like 1=’No’, 2=’Yes’; or 1=’Baseline’, 2=’Post treatment’) for each data element available from the form. In the example in Figure 2, the A0 form element 31d (A0e31d) reports the days between the base date and the day of surgery for the patient. In the corresponding A0.csv file column G lists the days between the base date and surgery for each patient.
Figure 2: In this example of extracting clinical data, the first step is to 1) find the form from the Form Dictionary, 2) Find the desired element and description in the accompanying form Dictionary (e.g. 6667_A0_Dictionary.csv) and finally 3) extract the values from the .csv data file.
The procedure above is basically how the statisticians organized the selected data for export, but the structure of the data dictionaries and individual forms are different for each clinical trial.
Acknowledgements
This shared data set was provided in collaboration with the American College of Radiology Core Lab. Many thanks are due to the ACRIN 6667 trial team, and all the patients participating in the study. This study was supported by American College of Radiology Imaging network (ACRIN), which received funding from the National Cancer Institute through UO1 CA080098, U01 CA190254 and R50 CA211270 (Muzi), under the American Recovery and Reinvestment ACT of 2009 (ARRA) and UO1 CA079778.
Please see QIN ECOG-ACRIN Data Sharing page for an overview and list of other ECOG-ACRIN data collections available on TCIA.
Related Publications
Publications by the Dataset Authors
The authors recommended the following as the best source of additional information about this dataset:
Publication Citation |
|
|
Lehman, C. D., Gatsonis, C., Kuhl, C. K., Hendrick, R. E., Pisano, E. D., Hanna, L., Peacock, S., Smazal, S. F., Maki, D. D., Julian, T. B., DePeri, E. R., Bluemke, D. A., & Schnall, M. D. (2007). MRI Evaluation of the Contralateral Breast in Women with Recently Diagnosed Breast Cancer. New England Journal of Medicine, 356(13), 1295–1303. https://doi.org/10.1056/nejmoa065447 |
.
Research Community Publications
TCIA maintains a list of publications that leveraged this dataset. If you have a manuscript you’d like to add please contact TCIA’s Helpdesk.